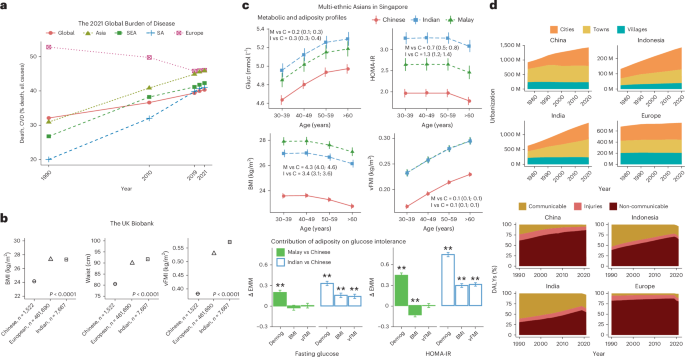
Adversity, adiposity, nutrition and metabolic well-being in multi-ethnic Asia
NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016).
Google Scholar
NCD Risk Factor Collaboration. Repositioning of the global epicentre of non-optimal cholesterol. Nature 582, 73–77 (2020).
Google Scholar
NCD Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980 (2021).
Google Scholar
International Diabetes Federation. IDF Diabetes Atlas 10th edn (2021).
International Diabetes Federation. IDF Diabetes Atlas 9th edn (2019).
Naghavi, M. et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2100–2132 (2024).
Google Scholar
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results (2020).
Caleyachetty, R. et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 9, 419–426 (2021).
Google Scholar
McLaren, J. et al. Weight gain leads to greater adverse metabolic responses in South Asian compared with white European men: the GlasVEGAS study. Nat. Metab. 6, 1632–1645 (2024).
Google Scholar
Mina, T. et al. Adiposity and metabolic health in Asian populations: an epidemiological study using dual-energy X-ray absorptiometry in Singapore. Lancet Diabetes Endocrinol. 12, 704–715 (2024).
Google Scholar
Jamal, R. et al. Cohort Profile: The Malaysian Cohort (TMC) project: a prospective study of non-communicable diseases in a multi-ethnic population. Int. J. Epidemiol. 44, 423–431 (2015).
Google Scholar
Vicks, W. S. et al. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: an electronic health record study. BMC Public Health 22, 1954 (2022).
Fazli, G. S., Moineddin, R., Bierman, A. S. & Booth, G. L. Ethnic differences in prediabetes incidence among immigrants to Canada: a population-based cohort study. BMC Med. 17, 100 (2019).
NCD Risk Factor Collaboration. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 569, 260–264 (2019).
Google Scholar
Pischon, T. et al. General and abdominal adiposity and risk of death in europe. New Engl. J. Med. 359, 2105–2120 (2008).
Google Scholar
Wong, M. C. S. et al. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 35, 673–683 (2020).
Google Scholar
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 16, 177–189 (2020).
Google Scholar
Nazare, J.-A. et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am. J. Clin. Nutr. 96, 714–726 (2012).
Google Scholar
Hales, N. & Barker, D. J. P. The thrifty phenotype hypothesis. Br. Med. Bull. (2001).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860 (2021).
Google Scholar
Kuchenbaecker, K. et al. The transferability of lipid loci across African, Asian and European cohorts. Nat. Commun. 10, 4330 (2019).
Giri, A. et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 51, 51–62 (2019).
Google Scholar
Suzuki, K. et al. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature 627, 347–357 (2024).
Google Scholar
Smith, K. et al. Multi-ancestry polygenic mechanisms of type 2 diabetes. Nat. Med. 30, 1065–1074 (2024).
Google Scholar
Mahajan, A. et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat. Genet. 54, 560–572 (2022).
Google Scholar
Wang, Y. et al. Polygenic prediction across populations is influenced by ancestry, genetic architecture, and methodology. Cell Genom. 3, 100408 (2023).
Google Scholar
Ge, T. et al. Development and validation of a trans-ancestry polygenic risk score for type 2 diabetes in diverse populations. Genome Med. 14, 70 (2022).
Google Scholar
Wang, X. et al. The Health for Life in Singapore (HELIOS) Study: delivering precision medicine research for Asian populations. Nat. Commun. 17, 1 (2026).
Google Scholar
Hodgson, S. et al. Genetic basis of early onset and progression of type 2 diabetes in South Asians. Nat. Med. 31, 323–331 (2025).
Google Scholar
Logsdon, G. A., Vollger, M. R. & Eichler, E. E. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 21, 597–614 (2020).
Google Scholar
Wu, Z. et al. Structural variants in the Chinese population and their impact on phenotypes, diseases and population adaptation. Nat. Commun. 12, 6501 (2021).
Genomics England. Long-Read Genomic Data (2025).
Brauer, M. et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2162–2203 (2024).
Google Scholar
Parthasarathi, S. K., Hebbani, A. V. & Dharmavaram Desai, P. P. Vegetarian ethnic foods of South India: review on the influence of traditional knowledge. J. Ethn. Food. 9, 42 (2022).
Google Scholar
Sasaki, S.; for Working Group 1 of the Healthy Diet Research Committee of International Life Sciences Institute, Japan. What is the scientific definition of the Japanese diet from the viewpoint of nutrition and health?. Nutr. Rev. 78, 18–26 (2020).
Google Scholar
Kim, S. H. et al. Korean diet: characteristics and historical background. J. Ethn. Food. 3, 26–31 (2016).
Google Scholar
Ma, G. Food, eating behavior, and culture in Chinese society. J. Ethn. Food. 2, 195–199 (2015).
Google Scholar
Harmayani, E. et al. Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal. J. Ethn. Food. 6, 1 (2019).
Google Scholar
Imai, T. et al. Traditional Japanese Diet Score — association with obesity, incidence of ischemic heart disease, and healthy life expectancy in a global comparative study. J. Nutr. Health Aging 23, 717–724 (2019).
Google Scholar
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results (2022).
Carioli, A., Schiavina, M. & Melchiorri, M. GHS-COUNTRY-STATS R2024A – GHSL country statistics by degree of urbanization, multitemporal (1975-2030). European Commission, Joint Research Centre (JRC) with major processing by Our World In Data (2024).
Roser, M., Ritchie, H. & Spooner, F. Burden of disease. Our World in Data (2021).
Mente, A. et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 5, 774–787 (2017).
Google Scholar
Harvard T.H. Chan School of Public Health. PURE study makes headlines, but the conclusions are misleading. The Nutrition Source (2017).
Mozaffarian, D. & Forouhi, N. G. Dietary guidelines and health – is nutrition science up to the task? BMJ 360, k822 (2018).
Google Scholar
Miller, V. et al. Global dietary quality in 185 countries from 1990 to 2018 show wide differences by nation, age, education, and urbanicity. Nat. Food 3, 694–702 (2022).
Google Scholar
Imamura, F. et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob. Health 3, e132–e142 (2015).
Google Scholar
Edefonti, V. et al. Reproducibility and validity of a posteriori dietary patterns: a systematic review. Adv. Nutr. 11, 293–326 (2020).
Google Scholar
Afshin, A. et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393, 1958–1972 (2019).
Google Scholar
Lane, M. M. et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. BMJ 384, e077310 (2024).
Google Scholar
Mendoza, K. et al. Ultra-processed foods and cardiovascular disease: analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies. Lancet Reg. Health Am. 37, 100859 (2024).
Baker, P. et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obesity Rev. 21, e13126 (2020).
Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2023 (2023).
Touvier, M. et al. Ultra-processed foods and cardiometabolic health: public health policies to reduce consumption cannot wait. BMJ 383, e075294 (2023).
Google Scholar
Braesco, V. et al. Ultra-processed foods: how functional is the NOVA system? Eur. J. Clin. Nutr. 76, 1245–1253 (2022).
Google Scholar
Atanasova, P. et al. Food environments and obesity: a geospatial analysis of the South Asia Biobank, income and sex inequalities. SSM Popul. Health 17, 101055 (2022).
Gaupholm, J., Papadopoulos, A., Asif, A., Dodd, W. & Little, M. The influence of food environments on dietary behaviour and nutrition in Southeast Asia: a systematic scoping review. Nutr. Health 29, 231–253 (2022).
Google Scholar
Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. (2020).
Internal Displacement Monitoring Centre. 2023 Global Report on Internal Displacement. (2023).
Global Network Against Food Crises. Global Report Food Crises. (2023).
Nagpaul, T., Sidhu, D. & Chen, J. The Hunger Report: an in-Depth Look at Food Insecurity in Singapore. (2020).
Karanja, A., Ickowitz, A., Stadlmayr, B. & McMullin, S. Understanding drivers of food choice in low- and middle-income countries: a systematic mapping study. Glob. Food Sec. 32, 100615 (2022).
Google Scholar
Mossenson, S. et al. The nutritional quality of food donated to a Western Australian Food Bank. Nutrients 16, 509 (2024).
Leung, C. W. et al. Food insecurity and ultra-processed food consumption: the modifying role of participation in the Supplemental Nutrition Assistance Program (SNAP). Am. J. Clin. Nutr. 116, 197–205 (2022).
Google Scholar
Yeo, N. These low-income families want to eat fresh, healthy food but it’s becoming costlier and charities are strapped for donations. Channels New Asia (2024).
Pelham-Burn, S. E., Frost, C. J., Russell, J. M. & Barker, M. E. Improving the nutritional quality of charitable meals for homeless and vulnerable adults. A case study of food provision by a food aid organisation in the UK. Appetite 82, 131–137 (2014).
Google Scholar
Daffu-O’Reilly, A. et al. Exploring the religious practice of langar as a route to health promotion in the Sikh community in Northern England: a qualitative study. J. Relig. Health (2024)
Eskandari, F., Lake, A. A., Rose, K., Butler, M. & O’Malley, C. A mixed-method systematic review and meta-analysis of the influences of food environments and food insecurity on obesity in high-income countries. Food Sci. Nutr. 10, 3689–3723 (2022).
Google Scholar
Brandt, E. J., Chang, T., Leung, C., Ayanian, J. Z. & Nallamothu, B. K. Food insecurity among individuals with cardiovascular disease and cardiometabolic risk factors across race and ethnicity in 1999-2018. JAMA Cardiol. 7, 1218–1226 (2022).
Google Scholar
Phelps, N. H. et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Google Scholar
Pourmotabbed, A. et al. Food insecurity and mental health: a systematic review and meta-analysis. Public Health Nutr. 23, 1778–1790 (2020).
Google Scholar
Blencowe, H. et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob. Health 7, e849–e860 (2019).
Google Scholar
Barker, D. J. In utero programming of chronic disease. Clin. Sci. 95, 115–128 (1998).
Google Scholar
Fall, C. H. D. Non-industrialised countries and affluence. Br. Med. Bull. 60, 33–50 (2001).
Google Scholar
Küpers, L. K. et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat. Commun. 10, 1893 (2019).
Yajnik, C. S. Early life origins of the epidemic of the double burden of malnutrition: life can only be understood backwards. Lancet Reg. Health Southeast Asia 28, 100453 (2024).
Google Scholar
Horikoshi, M. et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 538, 248–252 (2016).
Google Scholar
Nongmaithem, S. S. et al. Babies of South Asian and European ancestry show similar associations with genetic risk score for birth weight despite the smaller size of South Asian newborns. Diabetes 71, 821–836 (2022).
Google Scholar
Zhang, Y. et al. Association of large for gestational age with cardiovascular metabolic risks: a systematic review and meta-analysis. Obesity 31, 1255–1269 (2023).
Google Scholar
Do, W. L. et al. Epigenome-wide association study of diet quality in the Women’s Health Initiative and TwinsUK cohort. Int. J. Epidemiol. 50, 675–684 (2021).
Google Scholar
Chambers, J. C. et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 3, 526–534 (2015).
Google Scholar
Fraszczyk, E. et al. Epigenome-wide association study of incident type 2 diabetes: a meta-analysis of five prospective European cohorts. Diabetologia 65, 763–776 (2022).
Google Scholar
Agha, G. et al. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation 140, 645–657 (2019).
Google Scholar
Tan, W. L. W. et al. Epigenomes of human hearts reveal new genetic variants relevant for cardiac disease and phenotype. Circ. Res. 127, 761–777 (2020).
Google Scholar
Sayols-Baixeras, S. et al. Identification and validation of seven new loci showing differential DNA methylation related to serum lipid profile: an epigenome-wide approach. The REGICOR study. Hum. Mol. Genet. 25, 4556–4565 (2016).
Google Scholar
Juvinao-Quintero, D. L., Sharp, G. C., Sanderson, E. C. M., Relton, C. L. & Elliott, H. R. Investigating causality in the association between DNA methylation and type 2 diabetes using bidirectional two-sample Mendelian randomisation. Diabetologia 66, 1247–1259 (2023).
Google Scholar
Ott, R. et al. Epigenome-wide meta-analysis reveals associations between dietary glycemic index and glycemic load and DNA methylation in children and adolescents of different body sizes. Diabetes Care 46, 2067–2075 (2023).
Google Scholar
Lange de Luna, J. et al. Epigenome-wide association study of dietary fatty acid intake. Clin. Epigenetics 16, 29 (2024).
Loh, M. et al. Identification of genetic effects underlying type 2 diabetes in South Asian and European populations. Commun. Biol. 5, 329 (2022).
Reynolds, R. M. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology 38, 1–11 (2013).
Google Scholar
Lahti, M. et al. Maternal depressive symptoms during and after pregnancy and psychiatric problems in children. J. Am. Acad. Child Adolesc. Psychiatry 56, 30–39 (2017).
Yehuda, R. et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J. Clin. Endocrinol. Metab. 90, 4115–4118 (2005).
Google Scholar
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M. & Mondelli, V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21, 642–649 (2016).
Google Scholar
Seckl, J. R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151, U49–U62 (2004).
Google Scholar
Chapman, K., Holmes, M. & Seckl, J. 11Β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 93, 1139–1206 (2013).
Google Scholar
Wyrwoll, C. S., Holmes, M. C. & Seckl, J. R. 11β-Hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front. Neuroendocrinol. 32, 265–286 (2011).
Google Scholar
Mina, T. H. T., Räikkönen, K., Riley, S. C. S., Norman, J. E. J. & Reynolds, R. M. R. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: role of obesity and sex. Psychoneuroendocrinology 59, 112–122 (2015).
Google Scholar
Sharp, G. C. et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum. Mol. Genet. 26, 4067–4085 (2017).
Google Scholar
Küupers, L. K. et al. Maternal dietary glycemic index and glycemic load in pregnancy and offspring cord blood DNA methylation. Diabetes Care 45, 1822–1832 (2022).
Google Scholar
Howe, C. G. et al. Maternal gestational diabetes mellitus and newborn DNA methylation: mindings from the pregnancy and childhood epigenetics consortium. Diabetes Care 43, 98–105 (2020).
Google Scholar
Cardenas, A. et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: epigenome-wide associations at birth and persistence into early childhood. Clin. Epigenetics 11, 56 (2019).
Sharma, R. et al. Maternal–fetal stress and DNA methylation signatures in neonatal saliva: an epigenome-wide association study. Clin. Epigenetics 14, 87 (2022).
Kotsakis Ruehlmann, A. et al. Epigenome-wide meta-analysis of prenatal maternal stressful life events and newborn DNA methylation. Mol. Psychiatry 28, 5090–5100 (2023).
Google Scholar
Sammallahti, S. et al. Maternal anxiety during pregnancy and newborn epigenome-wide DNA methylation. Mol. Psychiatry 26, 1832–1845 (2021).
Google Scholar
Vehmeijer, F. O. L. et al. DNA methylation and body mass index from birth to adolescence: meta-analyses of epigenome-wide association studies. Genome Med. 12, 105 (2020).
Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541, 81–86 (2017).
Google Scholar
Jain, P. R. et al. Nuclear regulatory disturbances precede and predict the development of type-2 diabetes in Asian populations. Preprint at medRxiv (2025).
McAllan, L. et al. Integrative genomic analyses in adipocytes implicate DNA methylation in human obesity and diabetes. Nat. Commun. 14, 2784 (2023).
Google Scholar
Tinnion, R. et al. Preterm birth and subsequent insulin sensitivity: a systematic review. Arch. Dis. Child. 99, 362–368 (2014).
Google Scholar
Finken, M. J. J., Van Der Voorn, B., Heijboer, A. C., De Waard, M. & Van Goudoever, J. B. Glucocorticoid programming in very preterm birth. Horm. Res. Paediatr. 85, 221–231 (2016).
Google Scholar
Ohuma, E. O. et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet 402, 1261–1271 (2023).
Google Scholar
Grote, N. K. et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 67, 1012–1024 (2010).
Google Scholar
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150 (2022).
Google Scholar
Popejoy, A. B. & Fullerton, S. M. Genomics is failing on diversity. Nature 538, 161–164 (2016).
Google Scholar
Fatumo, S. et al. A roadmap to increase diversity in genomic studies. Nat. Med. 28, 243–250 (2022).
Google Scholar
Nagai, A. et al. Overview of the BioBank Japan Project: study design and profile. J. Epidemiol. 27, S2–S8 (2017).
Google Scholar
Chen, Z. et al. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 40, 1652–1666 (2011).
Google Scholar
Kim, Y. Han, B. -G. & the KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int. J. Epidemiol. 46, e20 (2017).
Google Scholar
Feng, Y. C. A. et al. Taiwan Biobank: a rich biomedical research database of the Taiwanese population. Cell Genom. 2, 100197 (2022).
Song, P. et al. Data Resource Profile: understanding the patterns and determinants of health in South Asians—the South Asia Biobank. Int. J. Epidemiol. 50, 717–718 (2021).
Google Scholar
Kanaya, A. M. et al. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 37, 1621–1628 (2014).
Google Scholar
Nazare, J. A. et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The International Study of Prediction of Intra-Abdominal Adiposity and its relationship withcardiometabolic risk/intra-abdominal adiposity. Am. J. Clin. Nutr. 96, 714–726 (2012).
Google Scholar
Scott, W. R. et al. Investigation of genetic variation underlying central obesity amongst South Asians. PLoS ONE 11, e0155478 (2016).
Low, D. Y. et al. Metabolic variation reflects dietary exposure in a multi-ethnic Asian population. Nat. Metab. 7, 1939–1954 (2025).
Google Scholar
Wong, E. et al. The Singapore National Precision Medicine Strategy. Nat. Genet. 55, 178–186 (2023).
Google Scholar
Chan, S. H. et al. Analysis of clinically relevant variants from ancestrally diverse Asian genomes. Nat. Commun. 13, 6694 (2022).
Google Scholar
link